solvent inaccessible: UPPER CASE X
solvent accesible: lower case x
alpha helix: red x
solvent accesible: lower case x
alpha helix: red x
beta strand: blue x
3 - 10 helix: maroon x
hydrogen bond to main chain amide: bold x
3 - 10 helix: maroon x
hydrogen bond to main chain amide: bold x
hydrogen bond to mainchain carbonyl: underline x
disulphide bond: cedilla
positive phi: italic x
disulphide bond: cedilla
positive phi: italic x
10 20 30 40 50
d1qd1a1 ( 2 ) sqlVeCvPnFSeGknqevIdaIsraVa----qTpgCvlldvdsgpstnRT
d1qd1a2 ( 181 ) flLaFNINLlstreqAhrIAldLreqggrLkkVqA-iGwyldeknlA
b bb aaaaaaaaaaa bb bbbbb bb
60 70 80 90 100
d1qd1a1 ( 48 ) vYtFvg-rPed--VVeGALnAAraAyqlIdMsrhhgehpRMGALDvCPFi
d1qd1a2 ( 233 ) QVsTnLldfevtGLhtVfeeTcreAqel--------------slpvvgSq
bbbbbb aaaaaaaaaaaaaa bbbbb
110 120 130 140 150
d1qd1a1 ( 95 ) pvrgVtmdeCvrCAqaFGqrLAeelgVPVYLygeAArtagrqslpaLrag
d1qd1a2 ( 269 ) Lvg----lVplkALldAAafYcekenl-fll-----------qdehrIrl
bb aaaaaaaaaaaaaaa aaaaa
160 170 180
d1qd1a1 ( 145 ) eyeaLpekLkqaewaPDfgpsafvpsWGATVAGArk
d1qd1a2 ( 303 ) VvnrLgLdsla----------pFkpk-----erIieylv
aaaaa
| Domain ID | Name | Family | Source | Domain | STRING-DB |
|---|---|---|---|---|---|
| Formiminotransferase domain of formiminotransferase-cyclodeaminase. | Formiminotransferase domain of formiminotransferase-cyclodeaminase. | Pig (Sus scrofa) [TaxId: 9823] | 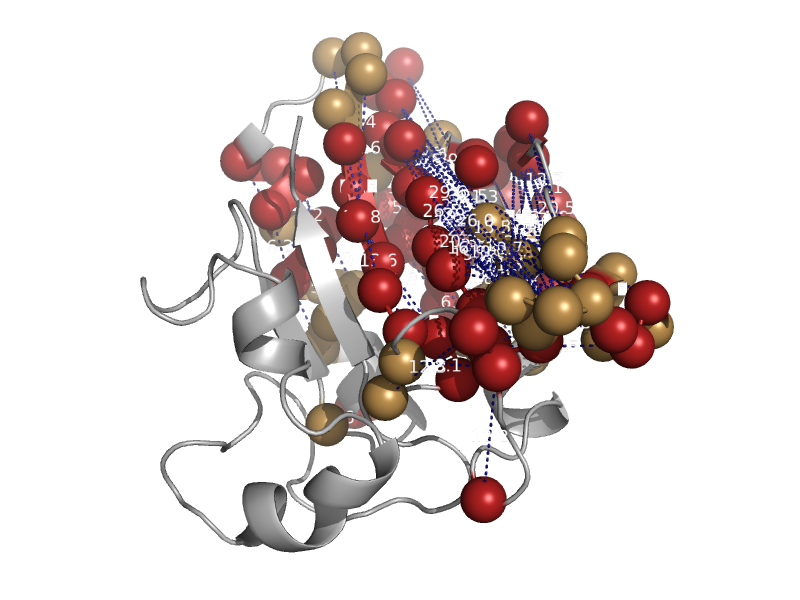 | view | |
| Formiminotransferase domain of formiminotransferase-cyclodeaminase. | Formiminotransferase domain of formiminotransferase-cyclodeaminase. | Pig (Sus scrofa) [TaxId: 9823] | 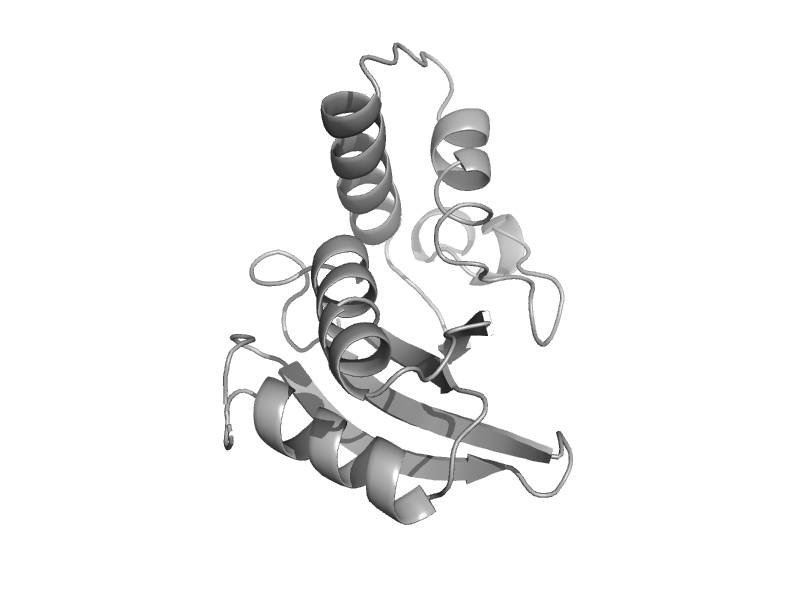 | view |
No outliers
| 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | 110 | 120 | 130 | 140 | 150 | 160 | 170 | 180 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | Q | L | V | E | C | V | P | N | F | S | E | G | K | N | Q | E | V | I | D | A | I | S | R | A | V | A | - | - | - | - | Q | T | P | G | C | V | L | L | D | V | D | S | G | P | S | T | N | R | T | V | Y | T | F | V | G | - | R | P | E | D | - | - | V | V | E | G | A | L | N | A | A | R | A | A | Y | Q | L | I | D | M | S | R | H | H | G | E | H | P | R | M | G | A | L | D | V | C | P | F | I | P | V | R | G | V | T | M | D | E | C | V | R | C | A | Q | A | F | G | Q | R | L | A | E | E | L | G | V | P | V | Y | L | Y | G | E | A | A | R | T | A | G | R | Q | S | L | P | A | L | R | A | G | E | Y | E | A | L | P | E | K | L | K | Q | A | E | W | A | P | D | F | G | P | S | A | F | V | P | S | W | G | A | T | V | A | G | A | R | K | - | - | - |
| - | - | - | F | L | L | A | F | N | I | N | L | L | S | T | R | E | Q | A | H | R | I | A | L | D | L | R | E | Q | G | G | R | L | K | K | V | Q | A | - | I | G | W | Y | L | D | E | K | N | L | A | Q | V | S | T | N | L | L | D | F | E | V | T | G | L | H | T | V | F | E | E | T | C | R | E | A | Q | E | L | - | - | - | - | - | - | - | - | - | - | - | - | - | - | S | L | P | V | V | G | S | Q | L | V | G | - | - | - | - | L | V | P | L | K | A | L | L | D | A | A | A | F | Y | C | E | K | E | N | L | - | F | L | L | - | - | - | - | - | - | - | - | - | - | - | Q | D | E | H | R | I | R | L | V | V | N | R | L | G | L | D | S | L | A | - | - | - | - | - | - | - | - | - | - | P | F | K | P | K | - | - | - | - | - | E | R | I | I | E | Y | L | V |
| Member | Chain | UniProtKB | GO term |
|---|---|---|---|
| d1qd1a1 | A | P53603 | GO:0005542 |
| d1qd1a1 | A | P53603 | GO:0016740 |
| d1qd1a1 | A | P53603 | GO:0005542 |
| d1qd1a1 | A | P53603 | GO:0016740 |
| d1qd1a2 | A | P53603 | GO:0005542 |
| d1qd1a2 | A | P53603 | GO:0016740 |
| d1qd1a2 | A | P53603 | GO:0005542 |
| d1qd1a2 | A | P53603 | GO:0016740 |
